Organoid Electroporation Email
Sonidel Limited works with pre-eminent Global researchers to deliver innovation and breakthrough research.
What differentiates us from our competitors is:
- Reproducible experimental results
- Innovative technology
- Low running costs
- Extensive Electroporation experience
- FREE TRIALS Before Purchase
- FREE Training and Support Service Post Purchase
NEPA21 Electroporation System
Compared to other devices on the market, the NEPA21 system offers the researcher a level of previously unavailable control over energy delivery to the electroporation target. This control is generated via:
- unique electroporation pulse-output configurations
- client-confirmed protocols and
- application-customised electrodes.
With this market-leading control and (user-independent) reproducibility of the technique, it is now possible to apply electroporation techniques to applications previously considered too sensitive for electroporation methodologies. One such application is Organoid Electroporation.
The NEPA21 is the only device on the market to approach Organoid Electroporation from the perspective of optimising delivered energy.
- The finer control over the delivered energy available with the NEPA21 offers specific and important advantages for organoid electroporation. As the thrust of NEPA21 protocols is to minimise delivered energy, this means that the targets are electroporated with less current (than competing device protocols).
- For particularly sensitive and delicate targets such as organoids, identifying and only delivering the required energy (and no more) to porate the membrane is of utmost importance for their viability post electroporation.
- The success of the NEPA21 for organoid electroporation is evident by the laboratories what have published with the NEPA21 system. Of note is the Organoid Group (previously Clever Group) at the Hubrecht Institute.
- The NEPA21 system is supported by a suite of over 250 different electrode configurations, which further enhance the applicability of the system and empower researchers with further experimental options and opportunities.
- With the NEPA21 system, the researcher can target both Dissociated Organoids (via the CU540 cuvette electrode) and Whole Organoids (via the CUY650P1 electrode).
NEPA21 TRIAL / DEMONSTRATION OFFER
Our confidence in the NEPA21 system is such that we offer all clients a FREE ‘TRY-BEFORE-YOU-PURCHASE’ TRIAL. The TRIAL is fully resourced with relevant TRAINING, PROTOCOL SUPPORT AND TROUBLESHOOTING. Indeed, via TEAMS/ZOOM, we can be present while you are testing with NEPA21 system with your research models.
If you would like to avail of this offer, please contact us on enquiries@sonidel.com .
Trial terms and conditions are straightforward. Please click here for further details: NEPA21 TRIAL Terms and Conditions
Organoid Electroporation
With the NEPA21, it is possible to electroporate both:
- Dissociated Organoids and
- Whole Organoids
Application-Customised Electrode Options
To further enhance the efficiency of the NEPA21 system, we developed application-specific electrodes.
For Dissociated Organoid Electroporation
- the CU540 Cuvette Holder and
- the EC-002S (2mm gap-cuvette) cuvette configuration
For the Whole Organoid Electroporation
- The CUY650P1 electrode
Dissociated Organoid Electroporation
| For this method, we recommend Cell Clusters rather than Dissociated Single Cells. We reference the following publication from one of our clients: Universal and Efficient Electroporation Protocol for Genetic Engineering of Gastrointestinal Organoids Technische Universität Dresden, Pape et al. (J Vis Exp. 2020 Feb 18;(156). doi: 10.3791/60704). For your information, this client reported to us: ”We reach very high transfection efficiencies each time and are still very happy to work with the Nepa21 electroporator. Thank you very much!” For our clients who avail of our TRIAL offer, we supply an Aide Memoire highlighting the critical success steps one needs to take extra care over when executing the protocol (for the first time). Notes: The client then tried the experiment using the Cas9 plasmid (10ug). For reference purposes, this is the previous protocol: In the Pape et al protocol, it has been shown that a regeneration time after electroporation of more than 10 min up to 40 min increases survivability and transfection efficiency especially of large plasmids. In test experiments, the same could be documented for organoids, leading to an incubation step of 40 min after electroporation in this protocol. The transfection results for GFP plasmids:
Another publication of note is: The optimised Electroporation Conditions: Poring Pulse (V = 175V, Pulse Length = 7.5 msec, Pulse Interval = 50 msec, Number of Pulse = 2 |
Whole Organoid Electroporation
| Whole Organoid Electroporation is performed in a Droplet held by a tweezers-type electrode. Of note is the following client publication:Probing the Tumor Suppressor Function of BAP1 in CRISPR Engineered Human Liver Organoids Cell Stem Cell. 2019 Jun 6;24(6):927-943.e6. doi: 10.1016/j.stem.2019.04.017. Epub 2019 May 23. Artegiani B, van Voorthuijsen L, Lindeboom RGH, Seinstra D, Heo I, Tapia P, López-Iglesias C, Postrach D, Dayton T, Oka R, Hu H, van Boxtel R, van Es JH, Offerhaus J, Peters PJ, van Rheenen J, Vermeulen M, Clevers H.Reference is made (in an earlier Clevers publication) to a Tweezer-type electrode. This is the electrode used in this publication and is our custom designed CUY650P1 electrode.The optimised Electroporation Conditions: Poring Pulse (V = 50V, Pulse Length = 10 msec, Pulse Interval = 50 msec, Number of Pulse = 4 Transfer Pulse (V = 20V, Pulse Length = 50 msec, Pulse Interval = 50 msec, Number of Pulse = 5 |
Other Notable Client Results
| Human and Mouse Gastric Organoids The National University of Singapore reports the following RESULTS:
(Client used our Nepa Gene plasmids, pCMV-EGFP, 4.8 kb)
|
| Modelling lung tumorigenesis using CRISPR/Cas9-based genome editing in ex vivo 3D organoids Note the online master thesis from the University of Applied Sciences Technikum Wien – Degree Program Tissue Engineering and Regenerative Medicine: Modeling lung tumorigenesis using CRISPR/Cas9-based genome editing in ex vivo 3D organoids (https://www.marshallplan.at/images/All-Papers/MP-2017/Weidinger+Pia_747.PDF)In 2.3.1. Electroporation section, the following comment is made. Initially, two different electroporation devices were tested, Amaxa 4D-Nucleofector and NEPA21. Transfection with Amaxa was performed according to the protocol for normal human bronchial epithelial cells from Lonza. For NEPA21, the protocol provided by Nepa Gene Co. for transfection of cell suspensions using cuvettes, version 6, was followed. Due to superior efficiency, NEPA21 was used for subsequent transfections. |
| Breast Cancer Organoids The protocol below was defined in the following publication: A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity Norman Sachs, Joep de Ligt, Oded Kopper, Ewa Gogola, Gergana Bounova, Fleur Weeber, Anjali Vanita Balgobind, Karin Wind, Ana Gracanin, Harry Begthel, Jeroen Korving, Ruben van Boxtel, Alexandra Alves Duarte, Daphne Lelieveld, Arne van Hoeck, Robert Frans Ernst, Francis Blokzijl, Isaac Johannes Nijman, Marlous Hoogstraat, Marieke van de Ven, David Anthony Egan, Vittoria Zinzalla, Jurgen Moll, Sylvia Fernandez Boj, Emile Eugene Voest, Lodewyk Wessels, Paul Joannes van Diest, Sven Rottenberg, Robert Gerhardus, Jacob Vries, Edwin Cuppen , Hans Clevers Cell. 2018 Jan 11;172(1-2):373-386.e10. doi: 10.1016/j.cell.2017.11.010. Epub 2017 Dec 7Optimised Electroporation Conditions: 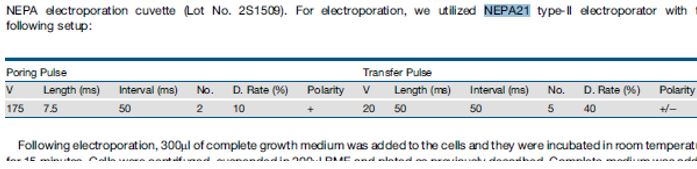 Interestingly, in this paper Opti-MEM was used instead of the BTXpress buffer. Interestingly, in this paper Opti-MEM was used instead of the BTXpress buffer. |
| Brain Organoid EP Note the following publication from the Weizmann Institute of Science, Rehovot, Israel Human Brain Organoids on a chip reveal the physics of Folding Eyal Karzbrun, Aditya Kshirsagar, Sidney R Cohen, Jacob H Hanna, Orly Reiner Nat Phys. 2018 May;14(5):515-522. doi: 10.1038/s41567-018-0046-7. Epub 2018 Feb 19. The publication demonstrates:
|
| Intestinal Organoids Note the following publication: Human Intestinal Organoids Maintain Self-Renewal Capacity and Cellular Diversity in Niche-Inspired Culture Condition Masayuki Fujii, Mami Matano, Kohta Toshimitsu, Ai Takano, Yohei Mikami, Shingo Nishikori, Shinya Sugimoto, Toshiro Sato Cell Stem Cell. 2018 Dec 6;23(6):787-793.e6. doi: 10.1016/j.stem.2018.11.016. The authors developed a modified culture condition for human intestinal organoids that improves the culture efficiency and maintains their long-term multi-differentiation capacity. scRNA-seq of human small intestinal crypts and organoids demonstrates that in vivo cellular diversity can be preserved in organoids cultured with the refined condition. |
| Whole Organoids in Cuvettes We assisted a client who wished to generate Cerebellar Organoids from human iPSCs and want to electroporate these organoids after 27 and 35 days with plasmids of his choice. The client wished to electroporate whole organoids in cuvettes. He chose the cuvette option because he wished to hit the outer cell layers of his organoids. He wanted to use 2mm gap cuvettes but was using wide-bore tips to place and extract the organoid but these tips did not fit in the 2 mm cuvettes. He did not want to use 4 mm gap cuvettes. Instead, we recommended he try a gel loading tip, similar to the following, which worked successfully with the 2mm gap cuvettes.  Optimised Electroporation Conditions Optimised Electroporation Conditions
|
NEPA21 Publications Link – Dissociated Organoids
NEPA21 Publications Link – Human Organoids
NEPA21 Full List of publications NEPA21 Cell Transfection Database
To request a trial or further information please contact us on enquiries@sonidel.com
Further information:
- NEPA21 List of publications , it lists relevant ex vivo Zygote publications (for Mouse/Rat/Marmosets/Pigs and Monkeys and in vivo Zygote (in the Oviduct – iGONAD Method) publications (for Mouse/ Rat and Hamster)
- Review of NEPA21 – a third-party (IDT) comparison of the NEPA21 against competing devices (GenePulser and Amaxa 4D) for Genome Editing Efficiency
- Kaneko – Genome Editing in Mouse and Rat by Electroporation – Chapter 7
- NEPAGENE zygotes genome editing methods
iPS/ES RESULTS
Illustrative client results for:
Publications:
Third-Party Testimony
Note these links:
- Charles River blog page promoting our NEPA21 system as the best in the field.
- The IDT website with the fully NEPA21 protocols for:
