Please contact enquires@sonidel.com for the step-by-step protocol.
Nannochloropsis Transformation
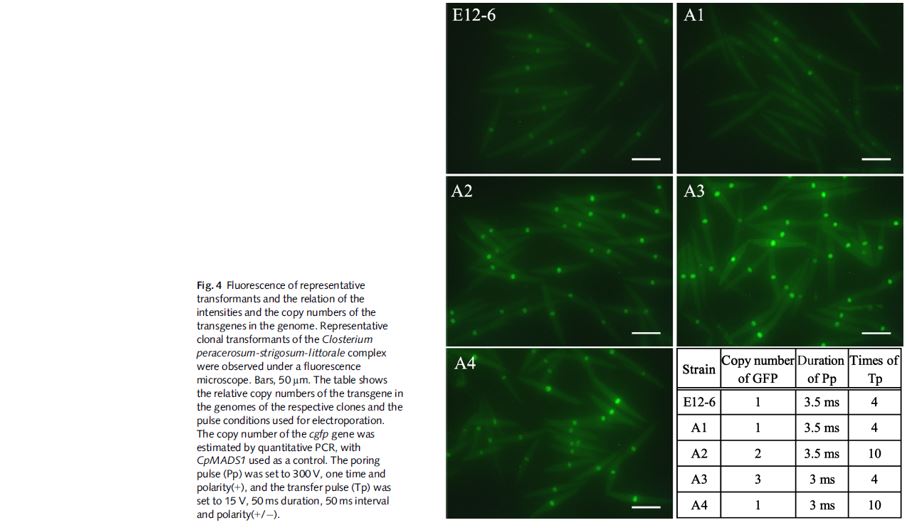
- Clients RESULTS from Hisoshima University
- The Hiroshima University researchers settled on condition 7, (1,750V) and reported arcing form 2,000V and higher
- Genome editing with removable TALEN vectors harbouring a yeast centromere and autonomous replication sequence in oleaginous microalga
- Sci Rep. 2022 Feb 15;12(1):2480.
- Target: Nannochloropsis cells (38uL)
- Materials: All-in-one TALEN-ARS vector (400ng)
- Electrode: 1mm gap cuvette
- Optimised EP Conditions: Pp. 1750V; 3.5ms; 50ms; 1pulse; +, Tp. 100V; 50ms; 50ms; 3 pulses; +/-
- 38 µL of cells and 400 ng of DNA of all-in-one PtTALEN-ARS targeting NoNR were mixed and placed into a 1 mm gap cuvette
- The cuvettes containing both cells and DNA were incubated at room temperature for 10 min. The cuvettes were then incubated on ice for 5 min.
- Electroporation was performed using an ELEPO21. The cells were quickly suspended in 5 mL of F2N liquid medium in a 15 mL plastic tube immediately after EP
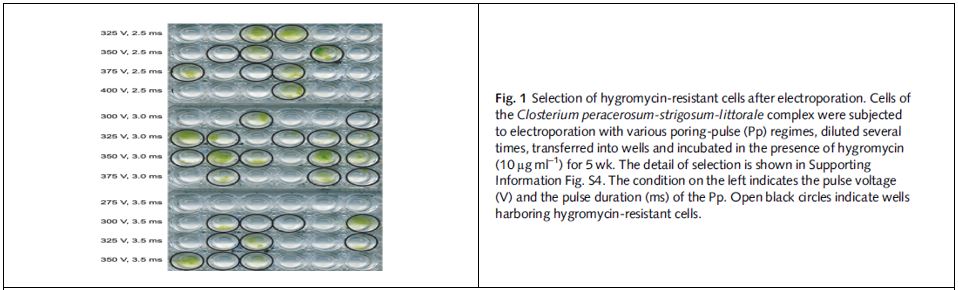
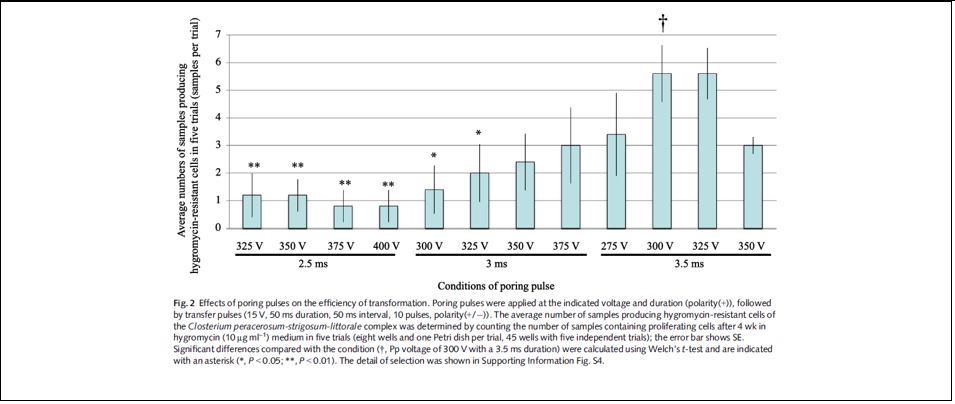
- The conditions were examined between 500-2000V. Under 1250-1750 V poring pulse voltage, approximately 1000 colony-forming units per µg DNA were obtained.
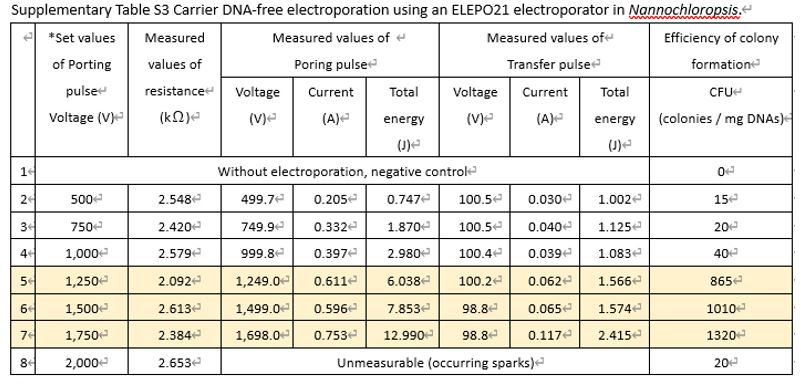
- To establish the transgene-free genome editing system, electroporation using carrier-DNAs, which are transgenes, was inconvenient because these carrier-DNAs remain in the host cells even after plasmid removal.
- However, carrier-DNAs are necessary for measurable and efficient electroporation using the Gene Pulser II.
Therefore, an electroporation system was established without carrier-DNAs using the highly efficient ELEPO21 (Supplementary Table S3 below).
Please contact enquires@sonidel.com for the step-by-step protocol.
Cell Transfection
Reference Histone functions as a cell-surface receptor for AGE
(Itakura, M., Yamaguchi, K., Kitazawa, R. et al., Nat Commun. 2022 May 27;13(1):2974).
J774A.1 cells were successfully transfected with either pcDNA4-H2B-IRES-GFP vector or empty pcDNA4 vector (mock) using an ELEPO21, incubated for 24 h, and subjected to flow cytometry analysis. The authors used the J774A.1 Mouse Macrophage-like cells to study the presence of cell-surface protein(s) that can bind to advanced glycation end products (AGEs).
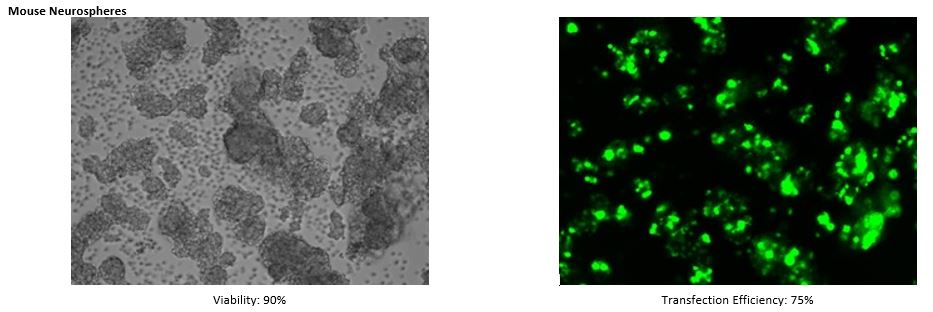
Transfection into Primary Cells
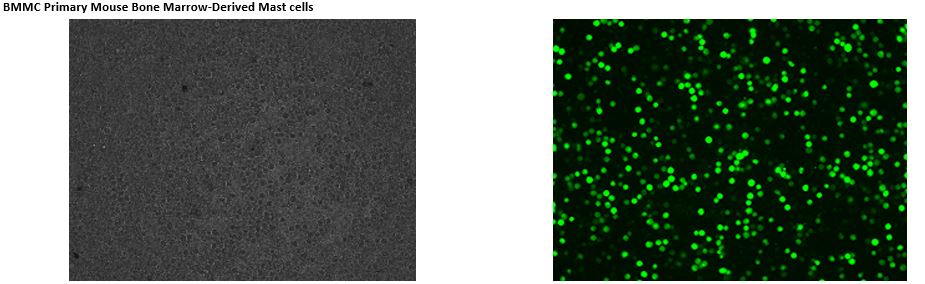
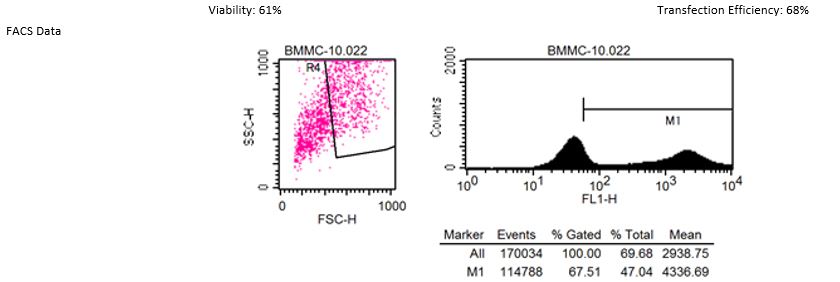
See MORE.
*The transfection data above are from the NEPA21 but we confirm that the ELEPO21 can achieve consistent results similar to the NEPA21.
Transfection into Stem Cells: ESCs, iPSCs and more
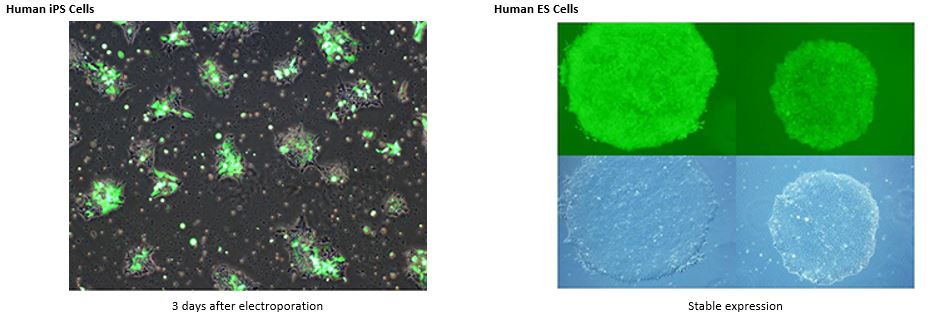 
See MORE.
*The transfection data above are from the NEPA21 but we confirm that the ELEPO21 can achieve consistent results similar to the NEPA21.
Transfection into Cell Lines
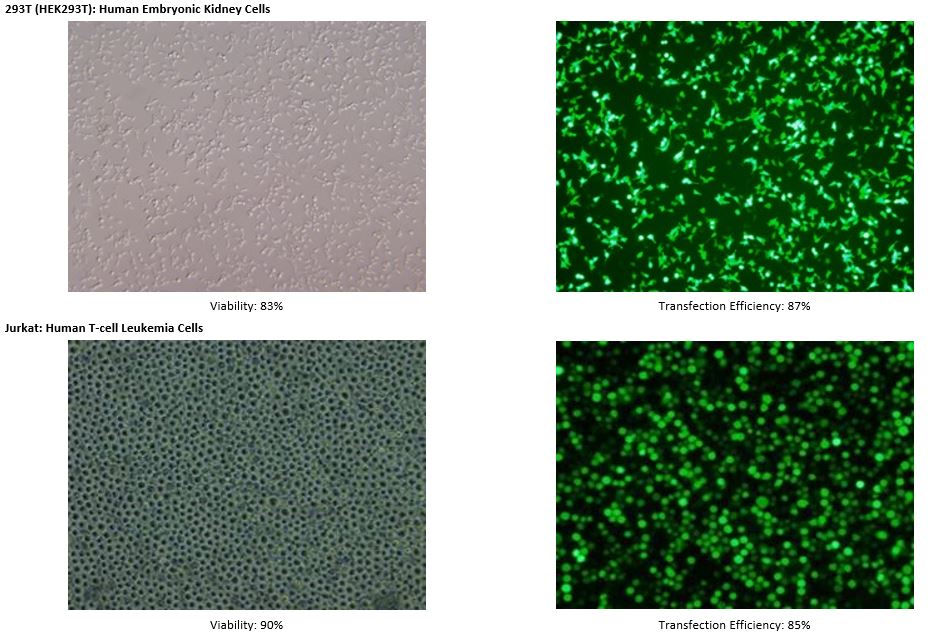
See MORE.
*The transfection data above are from the NEPA21 but we confirm that the ELEPO21 can achieve consistent results similar to the NEPA21.
| ELEPO21 Comparison with Competitors |
| No Special Buffers are required for the ELEPO21! |
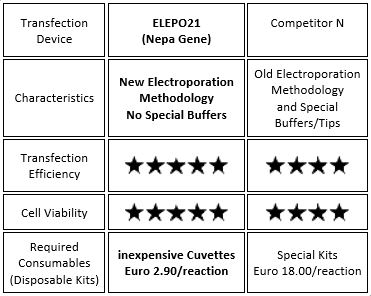 |
|
If your lab is still using a transfection device that requires expensive disposable kits/tips you need to consider the ELEPO21 system?
Researchers who previously used devices from competitors tell us that the low running cost of the ELEPO21 was a significant motivator in their decision to switch to the ELEPO21.
The running cost of ELEPO21 is significantly lower than other transfection devices!
Electroporation is a process by which one or more electrical pulses are delivered to a biological sample. These pulses result in the temporary disruption of the cell membrane, and under some conditions the cell wall. The “porated” cells are then able to quickly take up DNA, RNA, or proteins in the surrounding buffer.
Our ELEPO21 and NEPA Porator devices are designed to deliver multiple or up to two DC exponential decay waves with constant voltage. What makes them so special is their ability to first check the resistance of the sample via a trickle charge. We call this the ‘Ohm Check’. This pre-electroporation Ohm check procedure ensures that the samples’ buffering conditions are in the correct impedance range, in order to ensure one applies the correct amount of energy each and every electroporation. If the sample is not in the correct range, in many cases, a quick adjustment to the sample volume in the cuvette can bring resistance back into the optimal range, thereby enabling one to proceed with the delivery of the electric pulse. In addition, the Ohm Check process helps to identify “dirty” cells or DNA preps, so that these components can be re-purified and electroporation performed in the expectation of consistent results, from one electroporation to the next.
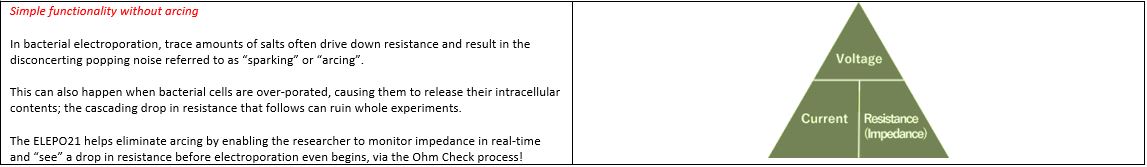
Each time the ELEPO21 delivers an electric pulse it follows the formula V = I x R (Ohms Law).
Precise Voltage Setting and Delivery
With the ELEPO21, one can be assured that the set voltage (and all other set electroporation parameters) is precisely delivered.
Oscilloscopic measurement readily confirms the precision of the ELEPO21 device.
But this is not the case for many competing devices. When connected to an oscilloscope, one will observe that the set parameters are not always what is delivered.
Impedance Fluctuation Control
Most competing devices do not account for resistance and/or resistance fluctuation from one sample to the next.
Sample resistance will fluctuate (from one sample to the next) due to several reasons: the volume/mass of the cells/tissue, the buffer used, etc.).
Most competing devices simply set a Voltage and pulse duration. They do not consider impedance fluctuation between samples.
Consider two samples, A and B where impedance fluctuates between samples and no intervention is taken of correct for the fluctuation.
If we set the same voltage for each sample(100V) but the Impedance of each sample varies (200 and 500 ohms respectively) the output Ampere will be different in each instance 0.5 and 0.2A respectively.

So, even though one sets the same conditions on the device (voltage and pulse duration), each sample receives a different Ampere. For this reason, the results in each sample will be different.
For competitor devices, this is the primary reason for inconsistent results from one experiment to the next. This inconsistency does not happen with the ELEPO21 system.
Unlike competing devices, the ELEPO21 accounts for impedance variability from one sample to the next by performing an ‘Ohm Check’ and measuring the sample impedance prior to electric pulse delivery.
If the measured impedance is not in the predetermined recommended range, it is manually manipulated into the predetermined recommended range.
Only then is the electric current applied.
In this instance:
- Voltage is controlled through the setting on the ELEPO21 device
- Impedance fluctuation (between samples) is corrected by performing an Ohm Check prior to pulse delivery (and ensuring the sample is in the correct range)
- In Ohms law (V = I x R) – V and R are precisely controlled which means that a controllable and constant current is delivered every electroporation
- Thus, if one has an optimised protocol, one can be sure one will obtain reproducible results every electroporation. One will not have to perform 10/15 electroporations to get 2/3 positive results.
- An added bonus of this electroporation methodology is that there will be no waste of expensive samples and more importantly researcher time.
Furthermore, the ELEPO21 delivers better RESULTS than competing devices.
It is cheaper to maintain and use as it does not require expensive buffers.
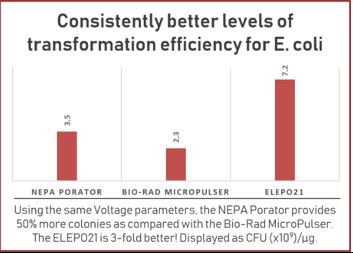
RESULTS – ELEPO21 and NEPA Porator Devices v Bio-Rad MicroPulser
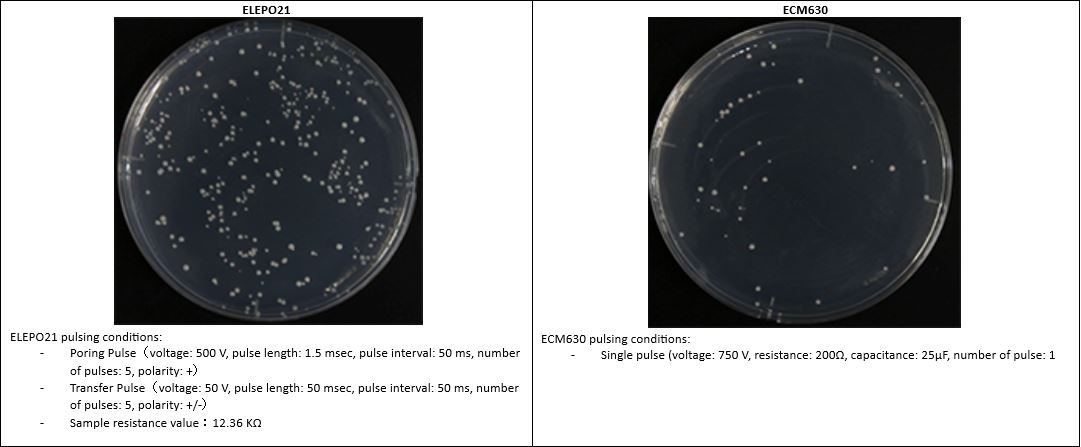
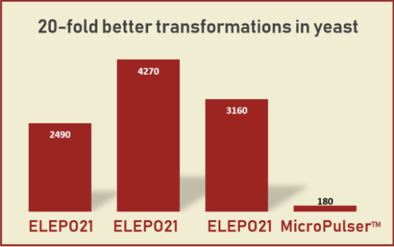
| RESULTS from labs in Japan illustrate the advantage of our ELEPO21 and NEPA Porator devices for E. coli and other bacterial cells.
The ELEPO21 and NEPA Porator’s unique ability to measure the electrical impedance of samples before pulsing, via the Ohm Check procedure, makes it suitable for a wide range of species.
Its pulse range is from 100V all the way up to 3,000V.
- 100V to 300V is perfect for the efficient transfection of many common lines of cultured cells.
- 800V to 3000V is ideal for organisms with a cell wall, such as bacteria, yeast, and other fungi.
Our ELEPO21 and NEPA Porator devices are used widely throughout Japan, and the data from these labs demonstrate that this system delivers exceptionally consistent results. |
 |

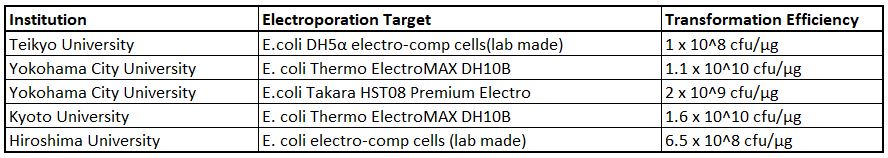
Note further data collected from other labs throughout Japan who have also recently evaluated the system with electro-competent E. coli cells:
ELEPO21 Impedance v Bio-Rad Capacitance
Bio-rad appears to prioritise the importance of capacitance over impedance in their protocols, and the ability of its capacitors to discharge energy quickly.
In terms of electroporation reproducibility and the viability of the electroporated target, we prioritise impedance.
The capacitance and impedance are not comparable nor similar.
That said, the Gene Pulser Xcell has an impedance measurement function (Bio-rad calls it “sample resistance” in their manual). But they do not consider it of critical importance to their protocols.
On the other hand, the ELEPO21 prioritises the critical significance of impedance measurement and its control for reproducible and successful electroporation.
A further contrast between the devices is:
- For the Gene Pulser Xcell, the impendance measurement range is: 5 ohms – 1,100 ohms, whereas
- For the ELEPO21, the impedance measurement range is: 1 ohms – 50,000 ohms.
|
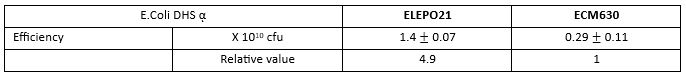 cfu: colony forming unit
cfu: colony forming unit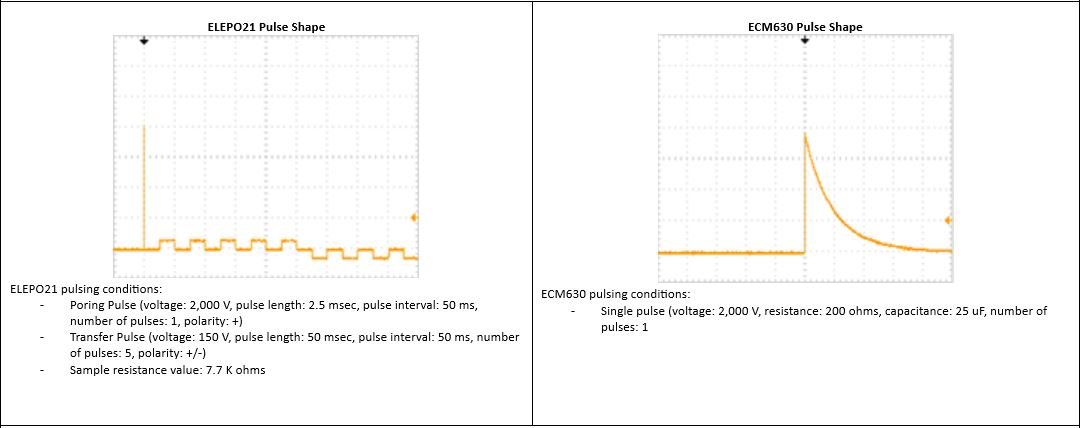
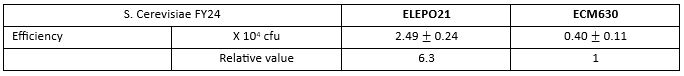 cfu: colony forming unit
cfu: colony forming unit